Ontario Animal Health Network (OAHN) Bovine Expert Network Quarterly Veterinary Report
Q2 Bovine Data from the Animal Health Laboratory
This summary has been compiled by Dr. Andrew Brooks, Animal Health Laboratory (AHL) from diagnostic submissions to the AHL Guelph and Kemptville locations.
A total of 1796 bovine cases were submitted to the AHL from May 1, 2024 to July 31, 2024. Of these, 156 submissions had a pathology component, consisting of 45 postmortem cases and 111 send-in cases (including 37 meat inspection cases). Overall, the diagnoses were routine, and no disease trends were identified.
Some cases of interest included two submissions involving congenital malformations and one case of mineral deficiency. Idiopathic craniosynostosis and marked cranial bone thickening were observed in a neonatal Angus calf. The postmortem examination revealed a domed head, thickening of the skull bones, and a patent foramen ovale in the heart. Another case involved dwarfism (chondrodysplasia) in a dairy calf. In this case, although the manganese concentrations in the liver were low, a genetic cause was not ruled out. Multiple mineral deficiencies were detected in a beef heifer from an organic herd that did not practice mineral supplementation. The animal had a small stature, was underweight, and analysis of the liver identified deficiencies of cobalt, copper, manganese, selenium, and zinc.
Salmonella Dublin continues to be detected in cattle. In this quarter, 195 bovine submissions had bacterial cultures (non-milk), and Salmonella spp. were isolated from 7 submissions (5 premises). The clinical problems included pneumonia, septicemia and enteritis.
Update on Influenza A(H5N1) in U.S. Dairy Cattle
Detections of highly pathogenic avian influenza (HPAI, H5N1 genotype B3.13) continue to be confirmed in U.S. dairy herds (Figure a, below). Recent detections have been concentrated in the Central Valley of California, bringing the number of confirmed affected states to 14 and the total number of confirmed herds to more than 300 (Figure a, b), though there is ongoing concern that the number of affected herds is greatly underreported for a variety of reasons. There have also been detections in poultry flocks, other livestock (alpacas), domestic animals (cats) and humans associated with infected cattle herds, emphasizing the broader implications of infected dairy premises.
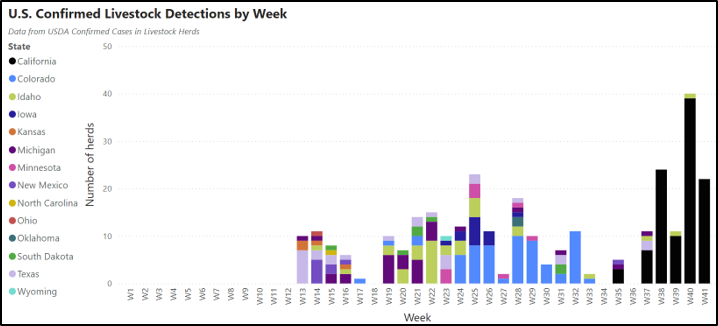
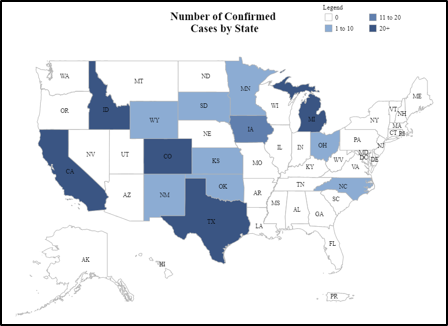
There have been no detections of HPAI in Canadian/Ontario cattle or other livestock species to date. However, infection pathways of concern remain, including contact with infected cattle (e.g., through importation or attending U.S. events), contact with contaminated vehicles or equipment (e.g., use of contaminated cattle transport trucks) and exposure to infected migratory birds. The USDA recently updated their National Epidemiological Brief on HPAI in dairy cattle with summarized data on transmission risks and clinical presentation based on data collected from affected herds.
To address some of this risk, the CFIA has changed import requirements for U.S. cattle and Canadian cattle returning to Canada following a short stay in the U.S. The latter involves mandatory isolation of returning animals, and testing requirements for those animals as well as the herd. Additional information on all relevant export/import policy is available online.
Biosecurity remains critical in preventing HPAI cases in Canadian livestock and other domestic animals. As such, until further notice, in addition to testing during suspect cases, CFIA will cover the fees for laboratory testing from samples submitted from non-clinical lactating dairy cows, and non-lactating dairy calves, heifers and dry cows (e.g., for testing newly acquired animals in isolation prior to integrating them with the herd). Additional information on sampling guidance can be found online.
Finally, through the fall season, practitioners are encouraged to remind their clients of best practices for reducing general influenza transmission in circumstances with increased risk:
- Fall fairs and exhibitions and other comingling events
- Premises with multiple species, or where staff or equipment are shared with other premises, especially those with poultry or swine
- Premises where humans have been diagnosed or are showing signs of influenza
Additional information on these topics can be found at:
- Biosecurity recommendations for Canadian fairs and exhibits – AHC News and Updates – Animal Health Canada
- Biosecurity for Canadian Dairy Farms: National Standard – inspection.canada.ca
- The flu | ontario.ca
- Animal health: Influenza | ontario.ca
- Highly pathogenic avian influenza (HPAI) in cattle – inspection.canada.ca
| What to do if you have suspicion* of a livestock case of HPAI: | What to do if you or your client find sick or dead birds or other wildlife on your property: |
| Producers – report clinical signs or suspected illness to your veterinarian immediately.
Veterinarians – contact your local CFIA animal health office if a high degree of suspicion.
*Criteria that contribute to a higher level of suspicion include: ·clinical presentation consistent with HPAI, without an alternative diagnosis ·an epidemiological link with a confirmed infected animal or herd/flock ·recent introductions into the herd within the last 30 days ·the discovery of dead or neurologic animals on the premises (for example, wild birds, raccoons, cats) ·potential for feed or water contamination by infected animals (for example, wild birds or mammals)
More information can be found online.
|
Report to the Canadian Wildlife Health Cooperative (CWHC) who may arrange for testing.
Phone: 866.673.4781 Email: on-nu@cwhc-rcsf.ca
|
Outbreak of Histophilus somni-Associated Bronchopneumonia in Dairy Calves at a Calf Rearing Facility
Dr. David Renaud, Ontario Veterinary College
Histophilus somni is a well-known pathogen in feedlot cattle, where it is frequently associated with respiratory diseases. However, its presence and impact in young dairy calves are far less commonly reported. This case study describes an outbreak of acute bronchopneumonia caused by Histophilus somni at a calf-rearing facility, resulting in a high mortality rate. The unusual occurrence of this pathogen in young dairy calves, may provide another pathogen to think about when dealing with respiratory disease in dairy calves.
The outbreak occurred at a calf-rearing facility housing 64 dairy calves. Over a period of 29 days, 12 out of 64 calves (18.8%) succumbed to respiratory disease. These calves were aged between 13 and 42 days at the time of death. Post-mortem examinations were conducted on all calves due to an ongoing research project revealing severe acute bronchopneumonia in the affected calves. Samples of lung tissue were submitted to the Animal Health Laboratory for bacterial culture. The results were somewhat unexpected, as 8 out of 12 (66.7%) calves had pure cultures of Histophilus somni isolated from their lungs. Further laboratory testing did not identify any additional respiratory pathogens, reinforcing the conclusion that Histophilus somni may have been the primary agent responsible for the bronchopneumonia in these calves. Following the initial outbreak, three additional calves in a subsequent group died, with Histophilus somni once again isolated from their lung samples.
In response to the outbreak, calves in the subsequent group were vaccinated with a Histophilus somni bacterin at arrival and 2 weeks after. Following vaccination, no additional mortalities or cases of respiratory disease were observed in the herd. However, it is unclear whether the vaccine was completely responsible for the drop in mortality as the calves were not from the same sources.
While Histophilus somni is not typically associated with young dairy calves, this case highlights the importance of considering it as a differential diagnosis in calf-rearing facilities when faced with severe outbreaks of respiratory disease. The pathogen is well-known in feedlot settings, but its ability to cause significant disease in young dairy calves may be under-recognized.
Seeking a New Bovine Network Member
The OAHN Bovine Network is currently seeking a veterinarian active in bovine veterinary medicine to join our team. We are looking for someone that has an interest in bovine health surveillance and wants to make a difference in the bovine industry by being a part of our network team.
The OAHN bovine network meets quarterly (4 times a year) virtually, for a 1.5-hour meeting. Some preparation time will be required in advance and possibly after each network meeting. The Network would be willing to tailor the needs of this role to the interests of the selected veterinarian. All involved veterinarians are compensated for their time.
If you are interested in being a network member on the OAHN Bovine Network, or would like more information on this role, please contact Cynthia Miltenburg at cynthia.miltenburg@ontario.ca.
OAHN Equine Project – Looking for Submissions
***Free testing for Strangles cases***
The OAHN equine network is beginning a project “Detection of Streptococcus equi subsp. equi infections in Ontario horses – A comparative study” and need your help.
The objective of this study is to compare the sensitivity and specificity of 2 real-time PCR tests and bacterial culture for the detection of S. equi subsp. equi in samples obtained from horses with suspected Strangles.
We are asking for samples (swab of pus or nasopharyngeal lavage or guttural pouch lavage) taken from horses with suspected Strangles to be submitted to the Animal Health Laboratory for testing. PCR and culture will be performed and then reported as per usual using the project submission form. At this time, please ONLY send samples from clinically affected horses.
Please note: Horses with positive S. equi test results will still be reported to OMAFA under the Animal Health Act, 2009 as per the usual process. Please contact Drs. Memo Arroyo (larroyo@uoguelph.ca) or Alison Moore (alison.moore@ontario.ca) with any questions.
Detections of Cryptosprodium parvum in Ontario 2017-2024
A recent discussion by the OAHN bovine expert network members prompted the question – has there been an increase in detections of Cryptospordium parvum in neonatal calves? To answer the question, we queried the Animal Health Laboratory (AHL) data to look for trends in detections of neonatal diarrhea pathogens over time.
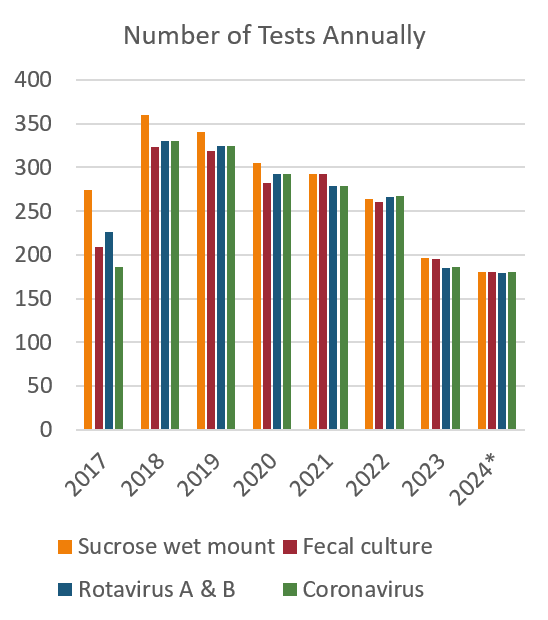
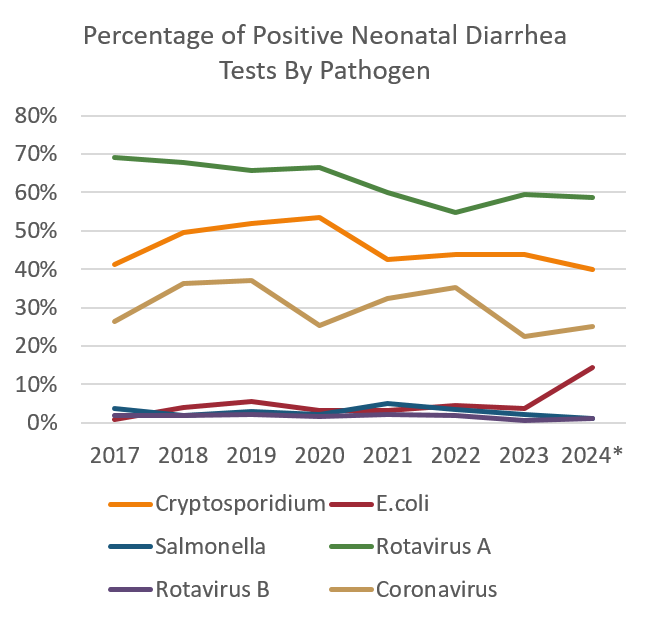
Positive results by test and total tests run were aggregated for Cryptospordiium parum detected by sucrose wet mount, E.coli and Salmonella spp detected by fecal bacterial culture, Rotavirus A and B by PCR test and Coronavirus by PCR test from submissions for calves 0 – 6 weeks of age. Data was collected from a 7-year period beginning 1 January 2014 until 30 September 2024.
The detections of C. parvum are relatively stable over the 7-year period. Rotavirus A and C.parvum tests had the highest proportion of positive results relative to the number of tests run annually. There were fewer tests run for all pathogens in 2023 and the available months of 2024 compared to the previous years. An upward trend in positive E.Coli results was noted in the 9-month period of 2024 included in the summary.
Thanks to Dr. Tanya Rossi for her time pulling the data to complete this summary.
*Includes 9 months (Jan to Sept)
Global Surveillance Update
Bluetongue Virus (BTV) Serotype 3 Outbreak in Europe
What first began as an outbreak in the Netherlands in September 2023, BTV Serotype 3 has now been detected in the Netherlands, Austria, Belgium, Czech Republic, Denmark, England, France, Germany, Luxembourg, Norway, Portugal, Spain, Sweden, Switzerland and Turkey in 2024. The initial virus source and introduction route in 2023 remains unknown, however since introduction, the virus has been spread by Culicoides spp biting midges and the movement of livestock.
Reports from GD Royal Netherlands indicate the clinical appearance in individual animals is variable but can include lesions in the mouth, tip of nose and udder, fever, conjunctivitis, red mucous membranes and lame animals with swollen coronary bands. Data analyses completed on 2023 Dutch cases showed a drop in milk production of 1 kg per cow per day for several weeks and poorer udder health on farms with BTV-3, and mortality among cattle aged over one year was twice as high on affected dairy and beef farms compared to unaffected farms.
The Agriculture and Horticulture Development Bord in the UK has an interesting webinar on the UK situation available at Bluetongue Virus – Update and Q&A | AHDB Webinar (youtube.com)
New Zealand’s “Mycoplasma Bovis Eradication Programme”
Prior to 2017, New Zealand remained the last major cattle-rearing country free from M. bovis infection. After its introduction, New Zealand embarked on an ambitious eradication program with the goals to reduce the impact of the disease for everyone and strengthen cattle biosecurity. The program and its progress is described in this M2 magazine article.
Have an idea for an infographic you’d like to see, or a podcast you’d like to hear? Reach out to a network member or email oahn@uoguelph.ca.


